Abstract
Background: Three CD19 directed CAR-T products have gained FDA approval for systemic large B-cell lymphoma. Due to heightened concerns of immune cell associated neurotoxicity syndrome (ICANS), patients with primary CNS lymphoma (PCNSL) were excluded from all pivotal CAR-T studies. Consequently, all three products carry a limitation of use in the PCNSL patient population per their FDA labels . Due to these exclusions, little is known about the treatment-related toxicities and therapeutic potential of the currently available CD19 directed CAR-T products in this challenging patient population with significant unmet need.
Methods: Based on our prior experience of tisagenlecleucel in secondary CNS lymphoma (PMID: 31320380) we conducted a pilot study with expansion of tisagenlecleucel in adults with relapsed or refractory PCNSL (NCT04134117). Patients had to be 18 years of age or older and have had progression or relapse following methotrexate-based therapy. All patients needed a confirmed diagnosis of PCNSL without evidence of systemic disease. Patients received a standard regimen of fludarabine (25 mg/m 2) and cyclophosphamide (250 mg/m 2) daily on days -5, -4, and -3 of infusion and a dose of 0.6-6.0 x 10 8 tisagenlecleucel CAR+ T-cells. Patients who had progressed on prior BTKi were allowed to continue given its beneficial effect on CAR-T expansion and function with cessation by month 3. The primary endpoint of this study was tolerability and toxicity including the rate and grade of CRS and ICANS per the 2019 ASTCT (American Society of Transplantation and Cellular Therapy) consensus criteria. Secondary endpoints included overall response rate and complete response rate to tisagenlecleucel per the international PCNSL Collaborative Group (IPCG) criteria which included MRI and CSF assessments. Exploratory endpoints included long-term efficacy, expansion, persistence and phenotype of tisagenlecleucel, cytokine profiling of the blood and CSF and CNS trafficking of CAR-T cells.
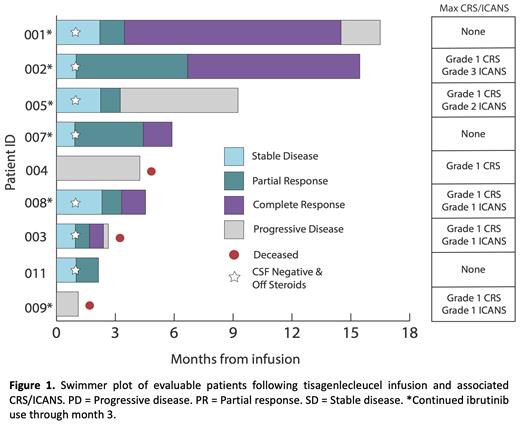
Results: As of April 1, 2021, 10 subjects (ages 35-70 years) were enrolled and 9 were infused with a median age of 67 years (range, 34-81). Of the 9 infused patients, the median time from leukapheresis to infusion was 30 days (range, 27-37). Patients were heavily pretreated prior to study enrollment and received a median of 4 prior lines of anti-neoplastic therapy. All patients had progressed or failed first line high-dose methotrexate (HD-MTX); two had a history of prior thiotepa based autologous stem cell transplant (ASCT). Eight out of 9 patients had progressed following a prior BTKi and/or an immunomodulatory drug (IMiD) as part of TEDDI-R (temozolomide, etoposide, doxil, dexamethasone, ibrutinib and rituximab, n = 3), ViPOR (venetoclax, ibrutinib, prednisone, obinutuzumab and Revlimid, n = 3), or as monotherapy (n = 5) and 2 patients had received prior stereotactic radiotherapy. All patients had measurable disease at time of lymphodepletion (pre-infusion). Grade 1 CRS was observed in 6 patients with a median onset of 4 days (range, 1-5) following tisagenlecleucel and no patients required intervention for CRS. ICANS developed in 5 out of the 9 patients, only a single case of grade 3 ICANS, with a median time of onset was 5 days (range, 3-11). With a median follow-up of 7.43 months for survivors, 6/9 patients were alive with 4/9 showing ongoing responses (Figure 1). Expansion of tisagenlecleucel was demonstrated in the peripheral blood and CSF. Nanostring and RNA pathway analysis of CSF infiltrates demonstrated higher degrees of CNS CAR-T penetration in responding patients and increased T-cell and macrophage gene signatures. Peripheral and CSF cytokines were assessed.
Conclusion: Tisagenlecleucel in r/r PCNSL was safe and efficacious in a highly refractory group of patients with significant unmet need. The majority of patients demonstrated a response per IPCG, including responses beyond 12 months. Tisagenlecleucel was found to expand in the peripheral blood and CNS with CSF gene signatures suggestive of higher CAR-T cell infiltrates in responding patients. Full trial safety data as well as additional follow-up and correlative studies will be presented.
Frigault: Editas: Consultancy; Iovance: Consultancy; Arcellx: Consultancy; Kite: Consultancy, Research Funding; Takeda: Consultancy; Novartis: Consultancy, Research Funding; BMS: Consultancy. Dietrich: Unum: Consultancy; Blue Earth Diagnostics: Consultancy; Magnolia: Consultancy; Gamaka Bio: Consultancy; Beacon Biosignals: Research Funding; Boehringer Ingelheim: Research Funding; BMS: Research Funding; Medimmune: Research Funding; Acerta: Research Funding; Orbus: Research Funding. Jordan: CereXis: Consultancy; Recursion: Consultancy; Navio Theragnostics: Consultancy. Forst: Eli Lilly: Current holder of individual stocks in a privately-held company. Plotkin: AstraZeneca: Consultancy; Akuous: Consultancy; NFlection Therapeutics: Other: Co-founder; NF2 Therapeutics: Other: Co-founder. Spitzer: Qihan Bio: Consultancy; Bluebird Bio: Consultancy; Jazz Pharmaceuticals: Consultancy; Syneos Health: Consultancy. Defilipp: Omeros, Corp.: Consultancy; Incyte Corp.: Research Funding; Regimmune Corp.: Research Funding; Syndax Pharmaceuticals, Inc: Consultancy. Maus: Tmunity: Consultancy; Novartis: Consultancy; Micromedicine: Consultancy, Current holder of stock options in a privately-held company; Kite Pharma: Consultancy, Research Funding; GSK: Consultancy; Intellia: Consultancy; In8bio (SAB): Consultancy; CRISPR therapeutics: Consultancy; Cabaletta Bio (SAB): Consultancy; BMS: Consultancy; Bayer: Consultancy; Atara: Consultancy; AstraZeneca: Consultancy; Astellas: Consultancy; Arcellx: Consultancy; Agenus: Consultancy; Adaptimmune: Consultancy; tcr2: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; century: Current equity holder in publicly-traded company; ichnos biosciences: Consultancy, Current holder of stock options in a privately-held company; Torque: Consultancy, Current holder of stock options in a privately-held company; WindMIL: Consultancy. Chen: Gamida: Consultancy; Incyte: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal